
Sign up for a new account.
And get access to
The latest T1D content
Research that matters
Our daily questions
Sign up by entering your info below.
Reset Your Password
Don't worry.
We will email you instructions to reset your
password.
This list provides, in alphabetical order of the manufacturer, the major connected insulin delivery devices currently FDA-cleared and available on the U.S. market or potentially available in the near future. All information provided has been obtained from and reviewed for accuracy by the manufacturer.
Manufacturer: Bigfoot Biomedical
Website: www.bigfootbiomedical.com
Product Name: Bigfoot Unity Diabetes Management Program
Description: The Bigfoot Unity System includes 3 primary components – two proprietary connected, smart pen caps compatible with all major U.S. brands of disposable insulin pens (one for basal insulin and one for rapid-acting insulin), a mobile app and the integrated continuous glucose monitor (iCGM) Abbott FreeStyle Libre2 or Bigfoot’s Bluetooth BGM. The system continuously translates monitored glucose data (BGM or CGM) into on-demand insulin dose recommendations based on individualized instructions from the person’s diabetes care provider. The dose recommendation and current glucose reading is accessible as it is displayed on the pen-cap screen. For more read T1D Exchange’s interview with Bigfoot CEO Jeffrey Brewer.
Management Assets: All-in-one solution (package) with the device + software + service; purchase from one entity; training and support on the system is provided
FDA status: Cleared 2021 for people with insulin-requiring diabetes
Intended population use: FDA-cleared for ages 12 years and older with T1D or T2D on multiple daily insulin injections (MDI)
Data integration/connectivity: Bigfoot Unity System is seamlessly integrated with Abbott’s FreeStyle Libre 2 iCGM sensor. The sensor communicates wirelessly to the Bigfoot Unity smart pen cap. When WiFi or cellular signal is present, the Bigfoot Unity pen cap transmits CGM and insulin dose data via Bluetooth to the phone where it is also uploaded wirelessly to the cloud-based Bigfoot Clinic Hub platform. The mobile app allows the user to view their data on their phone and share that data with loved ones and/or care partners.
Platform/app: Bigfoot Unity mobile app
Cost/coverage: This system is not available for purchase on its own. It is part of the holistic Bigfoot Unity Diabetes Management Program provided through the person’s diabetes care provider and there are no upfront costs.
What’s Next? Ongoing enhancements along with further development of their insulin delivery portfolio. In summer of 2021 Bigfoot Biomedical acquired the intellectual property assets of Common Sensing. As diabetes technology evolves, they will add new layers of technology where appropriate while still maintaining focus on simplicity, convenience, and ease of use.[/vc_column_text][/vc_column][/vc_row]

Manufacturer: Biocorp
Website: biocorpsys.com/en/our-products/connected-devices/mallya/
Product Name: Mallya
Description: A smart cap for all traditional (disposable) insulin pens that does not interfere with the workings of the pen. The smart cap is removable and can be reused for 2 years. Mallya automatically captures insulin injection data including dose, date and time.
Management Assets: Tracks each injection, the amount of insulin delivered and insulin type.
FDA status: Biocorp has submitted a Class II 510k application to FDA. Biocorp has received CE mark for a Class IIb medical device in Europe. Biocorp anticipates approval soon in Brazil.
Intended population use in U.S.: Adults 18 years and older
Data integration/connectivity: Sends data collected in real time to the software via Bluetooth
Platform/app: Biocorp has several partnerships that will leverage the Mallya platform:
- With Novo Nordisk to develop and distribute (if development is successful) a Mallya smart add-on device for the FlexTouch insulin pen
- With Amalgam RX using their iSage Rx app
- With Health to Sync (H2S) for use in Asia.
Cost/coverage: Not yet available in the U.S. Approximately 100 Euros (~$120 dollars).
What’s Next? New generation devices to cover additional pen platforms and significant reduction of size and improved form factor (shape).
Manufacturer: Collaboration and licensing agreement between Lilly and Welldoc
Website: Information not available
Product Name: Information not available. See description.
Description: Lilly is collaborating with Welldoc to advance their connected insulin solutions. In the system currently under development Lilly will integrate a new version of Welldoc’s BlueStar app into their pen platform to support personalized insulin dosing data while also providing users with insights to improve diabetes management. The new BlueStar app will integrate insulin dosing data for several Lilly insulins. In the first version of the pen platform, a data transfer module (Tempo Smart Button) will attach to the top of a prefilled, disposable insulin pen (Tempo Pen). When paired with the compatible app, the module will automatically transfer insulin dosing data. The platform integrates insulin, hardware, software, customer support and analytics to deliver personalized guidance.
Management Assets: Information not available.
FDA status: The prefilled, disposable insulin pen to be used in the first version of the pen platform was approved by the FDA in late 2019. Lilly plans to submit to the FDA in 2021 for the data transfer module, and Welldoc plans to submit to the FDA in 2021 for the new app.
Intended population use: Welldoc’s BlueStar app for use by adults with T1D and T2D.
Data integration/connectivity: CareLink software integrates all data; Smartphone connectivity allows viewing pump and CGM info and device notifications on phone, offer remote access to data to care partners; Bluetooth connectivity will allow for future software updates.
Data integration/connectivity: (see above under description)
Platform/app: Welldoc’s BlueStar
Cost/coverage: The pen platform is still in development. Lilly spokesperson stated, “We cannot speculate about the exact price or how these products might be reimbursed at this time.”
What’s Next? Lilly is also developing a fully disposable connected insulin pen to be used in future versions within the platform
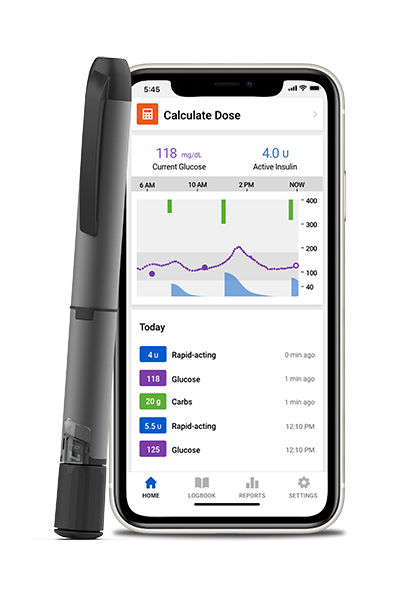
Manufacturer: Medtronic Diabetes
Website: www.medtronicdiabetes.com/products/inpen-smart-insulin-pen-system
Product Name: InPen with Insights by InPen diabetes management app system
Description: Durable (reusable) pen, uses a prefilled 300U cartridge of U100 rapid-acting insulin (cleared for Fiasp, Humalog, NovoLog) delivers bolus doses in 0.5U increments up to 30U per dose
FDA status: Cleared 2016, ages 12+; 2020 expanded clearance all ages; Additional 2020 clearance for 2 more meal modes in addition to carbohydrate counting
Intended population use: all ages (ages under 7 required supervision of an adult caregiver)
Management Assets: Reminds missed doses (basal and bolus), autoprime detection, dose calculator that is configured on individualized therapy settings, records all diabetes care data
Data integration/connectivity: Wireless transmission via Bluetooth to Insights by InPen (app), shareable with clinicians via fax, email; integrates with Guardian Connect CGM in real-time, Dexcom CGM G6, Bluetooth-enabled BGMs
Platform/app: Insights by InPen diabetes management app system
Cost/coverage: InPen is a prescription. People can obtain an InPen for as little as $35 per year.
What’s Next? Development of an InPen to deliver various basal insulins and integrate with the currently available InPen for bolus insulin delivery and the Insights by InPen app. In addition, Medtronic’s ultimate goal in connected insulin delivery is to close the loop for people who use MDI. Medtronic acquired Nutrino and Klue to enable the future of diabetes therapy through artificial intelligence. Nutrino helps us improve our predictive analytic capabilities around mealtimes and helps with the identification of what’s being consumed with greater accuracy. Klue’s gesture technology helps identify when that food is being consumed. We’re taking the best of AI technology, and our extensive CareLink database that contains over 500 million years of real-life data, with the goal of developing a best-in-class closed loop algorithm that we can leverage across our delivery platforms.
Manufacturer: NovoNordisk
Website: Information not available
Product Name: NovoPen Echo Plus
Description: The NovoPen Echo Plus is designed to support personalized management for people who take insulin using traditional durable pens. This pen is compatible with Fiasp and NovoLog 3ml Penfill cartridges. The pen records number of units dosed (displays doses in half unit increments to a maximum of 30U), time and date of all injected and priming doses. The memory display shows the amount of the last injection and time since the last injection. The Pen can integrate with partner apps and in-clinic solutions to view glucose levels and insulin doses side by side.
FDA status: Not yet filed
Intended population use: Not yet filed
Management assets: Information not available
Data integration/connectivity: Dose history can be viewed once the data is transferred wirelessly via near field communications (NFC), to a smartphone, tablet, or PC/Mac with a suitable app with software that supports upload of device data.
Platform/app: Integrations with several global partners are planned; exact platforms and rollout dates to be determined. See mention under Biocorp on partnership to add Mallya, the smart add-on device for the FlexTouch insulin pen).
Cost/coverage: Information not available
What’s Next? Information not available.







