The Future of T1D: Unannounced Meal Management Technology
Many people living with type 1 diabetes (T1D) use automated insulin delivery (AID) systems to help manage blood glucose levels….
A New App on the Market: Gluroo
Pump Users: Meet Gluroo Meet Gluroo, a new, free, collaborative diabetes management app that’s available for download. Gluroo,…
Here’s What You Need to Know About the Dexcom G7
The Dexcom G7 continuous glucose monitoring (CGM) system has been available in the U.S. since early 2023, and people are…
The Impact of Using a CGM Within Six Months of Your T1D Diagnosis
At this year’s American Diabetes Association 83rd Scientific Sessions, T1D Exchange presented three oral presentations, two panel discussions, and fifteen…
How the iLet ACE Closed-Loop Insulin Pump is So User-Friendly
The iLet ACE insulin pump and iLet Dosing Decision Software from Beta Bionics were approved by the FDA on May…
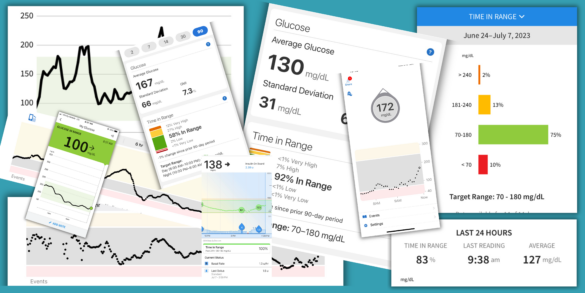
Time-in-Range: Making the Most of Your CGM Data
Disclaimer: T1D Exchange and the author are not medical professionals. Your target time-in-range may be tailored to your current needs…
All About the Medtronic Minimed 780G Closed-Loop Automated Insulin Pump System
Medtronic’s Minimed 780G hybrid closed-loop system received FDA approval on April 21, 2023, for use in people ages 7 and…
What’s New in Dexcom’s G7 Continuous Glucose Monitor
The Dexcom G7 continuous glucose monitor (CGM) received FDA clearance in December 2022 for people with type 1, type 2,…
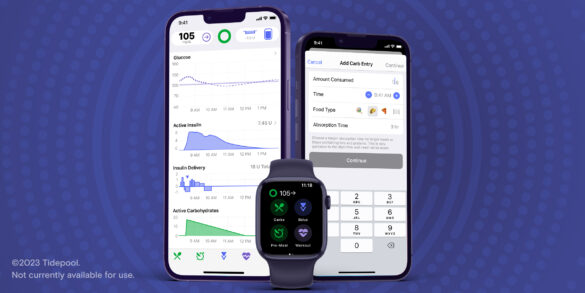
Tidepool Loop FDA Clearance: Chatting with CEO Howard Look
This week’s FDA clearance of the new Tidepool Loop iPhone app means “looping” could be even simpler and likely far…
Connected Insulin Delivery Devices
This list provides, in alphabetical order of the manufacturer, the major connected insulin delivery devices currently FDA-cleared and available on…