
Sign up for a new account.
And get access to
The latest T1D content
Research that matters
Our daily questions
Sign up by entering your info below.
Reset Your Password
Don't worry.
We will email you instructions to reset your
password.
This list provides information on the major AID systems currently approved for use by the US FDA. Information provided has been obtained from and reviewed for accuracy by the manufacturer or entity
Last updated January 2023

Manufacturer: Insulet Corporation
Website: https://www.omnipod.com/hcp/products/innovation
Product Name: Omnipod 5 Automated Insulin Delivery System
Description: Fully on-body AHCL/AID system; Dexcom G6 transmitter communicates directly to a wearable insulin-filled Pod; Personalized Model Predictive Control (MPC) algorithm is built into the Pod; Insulet-provided Controller or compatible personal smartphone* is used to start and stop Automated Mode, deliver boluses, view data, and change settings. Customizable Target Glucose from 110-150 mg/dL in 10 mg/dL increments, adjustable by time of day.
FDA status: Received FDA 510(k) clearance in Q1 of 2022. Full market release later in 2022.
FDA Intended (indications for) use: The Omnipod 5 ACE Pump can reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The Omnipod 5 ACE Pump is intended for single-person use and requires a prescription. SmartAdjust technology is intended for use with compatible iCGM and ACE pump to automatically increase, decrease, and pause delivery of insulin based on current and predicted glucose values in the management of T1D in persons 6 years of age and older. The Omnipod 5 SmartBolus Calculator calculates a suggested bolus dose based on user-entered carbohydrates, most recent sensor glucose value (or blood glucose reading if using fingerstick), rate of change of the sensor glucose (if applicable), insulin on board (IOB), and programmable correction factor, insulin to carbohydrate ratio, and target glucose value.
Unique assets: Activity feature (for times of decreased insulin needs, such as exercise). Waterproof. Discreet insulin delivery. Controller or compatible smartphone does not need to be near the Pod for the system to deliver automated basal delivery in Manual Mode and micro-boluses in Automated Mode.
Data integration/connectivity: Dexcom G6 transmitter communicates directly to Pod; pod communicates wirelessly to the Omnipod 5 App on Controller or compatible smartphone; Dexcom G6 transmitter communicates wirelessly to the Dexcom G6 app; Controller or compatible smartphone controls and monitors Pod operations with Bluetooth wireless technology.
Food coverage: Users will enter the amount of carbohydrates they are consuming into the SmartBolus calculator.
Platform/app: Omnipod 5 App
Cost/coverage: Omnipod 5 will launch at price parity to Omnipod DASH. Insulet plans to expand its “30 days of freedom” free trial period to new Omnipod 5 users. Omnipod 5 will be available exclusively through some pharmacies.
*For a list of compatible smartphone devices, visit https://www.omnipod.com/current-podders/resources/omnipod-5/device-compatibility
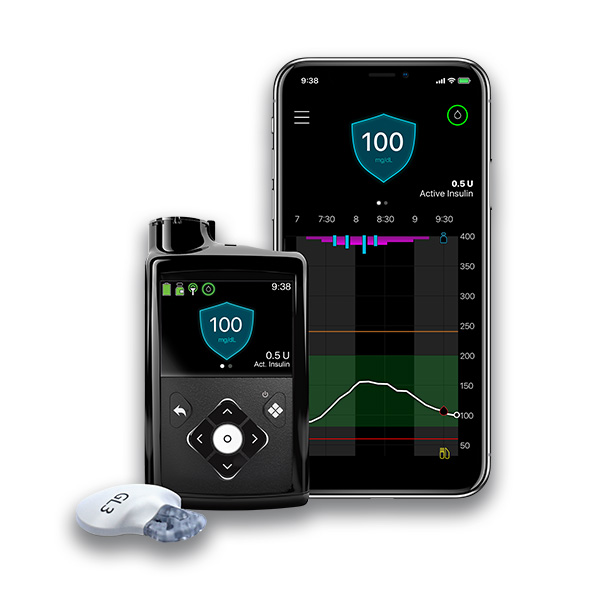
Manufacturer: Medtronic Diabetes
Website: https://www.medtronicdiabetes.com/products/minimed-780g-insulin-pump-system
Product Name: MiniMed 780G* — referred to by Medtronic as an advanced hybrid closed loop [AHCL] system or hybrid closed loop (HCL) system.
Description: AHCL/AID, the SmartGuard algorithm automates insulin delivery in a real-time adaptable way with predictive personalized diagnostics.
FDA status: Approved in April 2023
FDA Intended use: For people with type 1 diabetes ages 7 and older
Unique assets: Automatically adjusts background insulin with correction boluses every 5 minutes based on integrated CGM results, allows for adjustable target settings (to 100 mg/dL), assists with not forgetting to dose for meals, dosing for a meal late, or underestimating the carbohydrate content of meals.
Data integration/connectivity: Works with the Guardian 4 sensor and SmartGuard technology. CareLink software integrates all data; Smartphone connectivity allows viewing pump and CGM info and device notifications on the phone, and offers remote access to data to care partners; Bluetooth connectivity will allow for future software updates.
Platform/app: CareLink software
Future: The Personalized Closed Loop (PCL) insulin pump system, will be more advanced than MiniMed 780G was given a fast-track FDA process referred to as the Breakthrough Devices Program. PCL is in feasibility trials now. It will leverage the power of Klue’s (an entity acquired by Medtronic in 2019) gesture technology to ID when food is being consumed coupled with Nutrino, another entity that Medtronic acquired in 2018, which improves predictive analytic capabilities around mealtimes to deliver a higher level of personalized precision to dosing intelligence.
Cost/coverage: Pricing information coming soon.

Manufacturer: Tandem Diabetes Care
Website: https://www.tandemdiabetes.com/
Product Name: t:slim X2 with Control-IQ (t:slim X2 also available alone or with basal-IQ), NextGen product (see Future below).
Description: AHCL/AID; Predicts and helps prevent hypo-and hyperglycemia; uses CGM (Dexcom G6) data to hold glucose in range of 70-180 mg/dL (defined TiR); Auto-correct bolus doses; optional settings for sleep and exercise.
FDA status: Cleared 2019, ages 14+, Cleared ages 6+ 2020.
FDA Intended use: Basal-IQ and Control-IQ cleared by FDA as an interoperable automated glycemic controller (iAGC).
Unique assets: Free updatable software uploads for next-generation software with prescription; training and online tutorials required.
Data integration/connectivity: Integrated with Dexcom G6 CGM.
Platform/app: Users can utilize t:connect to make their mobile phone a discreet secondary screen from which to access pump data and settings, and for uploading pump data at their clinician visits. Expected summer 2022, users will have the ability to bolus without having to touch the t:slim X2 insulin pump. Learn more.
Cost/coverage: t:slim X2 with Control-IQ covered by most healthcare plans under the durable medical equipment (DME) deductible. t:slim X2 is covered by Medicare.
Future: Tandem considers Mobi (previously named t:sport mini-pump a next-generation device. It will be a discreet tubed pump (micropump) with no screen. It will contain an AID algorithm that will be controlled from the user’s mobile phone. At this point, there is no solid timeline for FDA submission or launch of this system.
This list provides information on several AID systems still in development in or outside the US. A few systems are joint ventures between several entities as described in the details. Information provided has been obtained from and reviewed for accuracy by the manufacturer or entity or other sources as noted.

Manufacturer: Beta Bionics, Inc. (a public benefit company & B Corp™)
Website: https://www.betabionics.com/
Product Name: iLet® Bionic Pancreas
Description: The iLet is a pocket-sized, wearable investigational medical device designed to autonomously dose insulin and/or glucagon. It is designed to be worn like an insulin pump; however, iLet users would enter only their body weight to initialize therapy and would not set any insulin regimen parameters. The iLet is designed to then automatically titrate and infuse insulin and/or glucagon doses without requiring the user to count carbohydrates, set insulin-to-carbohydrate ratios, set insulin basal rates, set correction factors, or determine bolus insulin for meals or corrections. CAUTION: The iLet® Bionic Pancreas is an investigational device limited by Federal (or United States) law to investigational use. Not available for sale.
FDA status: Investigational device. In 2021, the Company announced the completion of the randomized controlled trial for the Insulin-Only Bionic Pancreas Pivotal Trial. The Insulin-Only Bionic Pancreas Pivotal Trial was a multi-center, randomized controlled trial funded, in part, by a grant from the National Institutes of Health to Boston University, and conducted in collaboration with the IDE sponsor and study coordinator, the Jaeb Center for Health Research, and 16 clinical research centers across the U.S. It was designed to test the safety and efficacy of the insulin-only configuration of the iLet Bionic Pancreas System relative to a standard-of-care control group over a 13-week study period. The trial was conducted in the home-use setting and enrolled approximately 440 adults and children 6 years and older with type 1 diabetes. It was the largest sample size to date for a randomized controlled trial of an automated insulin delivery system. The study results have been included in a comprehensive submission package to the FDA for regulatory review of the Insulin-Only system.
Intended use: TBD by FDA
Data integration/connectivity: TBD
Platform/app: TBD

Manufacturer(s): Roche, Diabeloop, and Dexcom
Website: https://www.dbl-diabetes.com
Product Name: Accu-Chek Insight with DBLG1
Description: Roche teamed up with the French medtech company Diabeloop to take the first step into AID. People can use the Accu-Check Insight insulin pump with the AID system DBLG1 from Diabeloop. This is the first pump with a pre-filled cartridge that can be operated in loop mode. Pairing the Accu-Check Insight pump with the self-learning algorithm in DBLG1 aims to automate and personalize diabetes management. Every five minutes a glucose reading from the CGM is sent to the handset via Bluetooth. The algorithm analyses data in real-time and accounts for the physiology, history, and data entries to determine the correct insulin dose that is then administered by the insulin pump.
FDA status: No application filed to date
Approval Outside US: As of March 2021 it is available in Germany, Spain, Italy, the Netherlands, and Switzerland. Website contains country-specific information.
Intended use: The system requires a prescription and is intended for people with T1D 18 years of age and older.
Data integration/connectivity: DBLG1 system is a hybrid closed-loop system. Its core element is a self-learning algorithm (see description).
Platform/app: Diabeloop developed the YourLoops web platform for data visualization. All data recorded by DBLG1 is uploaded to the platform. Data can be shared with HCPs or family member.
Cost/coverage: Depends on the healthcare system in each country where the system is available.
Entity: Tidepool, a nonprofit organization
Website: https://www.tidepool.org/automated-insulin-dosing
Product Name: Tidepool Loop (this is not a standalone AID system). The goal is to make it interoperable (see Glossary with as many ACE pumps and iCGMs as possible. Announced partners to data include Medtronic, Insulet, and Dexcom.
Description: Tidepool Loop intends to be delivered as an iOS app that is available in the App Store with a prescription.
FDA status: Tidepool Loop was submitted to FDA as an iAGC and is currently under review.
FDA Intended use: More information expected after FDA review is complete.
Unique assets: Interoperability with other ACE pumps and integrated CGMs. More information expected after FDA review is complete.
Data integration/connectivity: More information expected after FDA review is complete.
Platform/app: Tidepool Loop is being developed as an iOS app.
Cost/coverage: No detail on cost or coverage are available currently.
Manufacturer(s): CamDiab*
Website: https://camdiab.com/
Product Name: CamAPS FX
Description: CamAPS FX uses an Android app (including smartphone pump control), Dexcom G6, and SOOIL Dana Diabecare RS pump or the Dana-i pump. The algorithm modulates insulin delivery every 8-12 minutes, requires a “simple setup” of body weight and total daily dose, and is “highly adaptive” based on daily insulin needs, after meal insulin needs independent of the pump basal rate, insulin-to-carbohydrate ratio and correction factor settings. The algorithm is housed on the app, which means that users need to keep the app in range of the system to stay in closed loop.
FDA status: No application submitted at present.
Approval Outside US: University of Cambridge spinoff CamDiab’s AID system is named CamAPS FX. It was CE-Marked (European Union) and launched in the UK in March 2020. Current availability/use is limited.
Intended use: People with T1D, aged 1 year and older
Data integration/connectivity: Data can be sent automatically from the app to Glooko and to Diasend (owned by Glooko). This allows for efficient data sharing with providers and caregivers.**
Platform/app: N/A
Cost/coverage: For costing details, click on “orders” on the website.
* Several attempts were made to contact this entity. Information above is from several sources including the CamDiab website and Close Concerns KnowledgeBase updated spring 2022.
** In March 2022 Ypsomed announced a partnership with CamDiab to develop an integrated AID system with Ypsomed’s myLife YpsoPump, CamDiab’s CamAPS FX AID algorithm and Dexcom G6 CGM.
Manufacturer(s): Ypsomed, Dexcom and Lilly*
Website: N/A
Product Name: Marketed in Europe as the mylife™ YpsoPump®
Description: YpsoPump, is small in size and has an icon-based touch screen. It will use a Dexcom CGM.
FDA status: Ypsomed plans to submit its pump for FDA clearance in an AID system in 2023. If cleared, Lilly will have exclusive rights to commercialize the pump in the U.S.
Approval Outside US: N/A
Intended use: N/A
Data integration/connectivity: N/A**
Platform/app: N/A
Cost/coverage: N/A
* Several attempts were made to contact this entity. Information above is from several sources including the website and Close Concerns Knowledge Base which was updated spring 2022.
** In March 2022 Ypsomed announced a partnership with CamDiab to develop an integrated AID system with Ypsomed’s myLife YpsoPump and CamDiab’s CamAPS FX AID algorithm and Dexcom G6 CGM. 3
References:
- Medtronic Acquires Klue. https://news.medtronic.com/2019-12-17-Medtronic-Acquires-Klue Accessed April 22, 2022.
- Medtronic to Acquire Nutrino Health. https://news.medtronic.com/2018-11-21-Medtronic-to-Acquire-Nutrino-Health Accessed April 22, 2022.
- Ypsomed partners with CamDiab Ltd to drive on smartphone based adaptive automated insulin delivery (AID) https://www.ypsomed.com/en/media/details/ypsomed-partners-with-camdiab-ltd-to-drive-on-smartphone-based-adaptive-automated-insulin-delivery-aid.html. Accessed April 21, 2022.







