Falla de la bomba de insulina: un farmacéutico especializado en diabetes explica los próximos pasos
The original article was written by Diana Isaacs and translated into Spanish by Hannah Doskicz. Las tasas de uso…
Then & Now: T1D Tech in 6 Years of the T1D Exchange Registry
We’re celebrating six years of the T1D Exchange Registry! Since its online launch in 2019, we’ve gathered the stories of…
Reducing Requirements for Insulin Pump Referral Improves Pump Initiation for Publicly Insured Children and Youth With Type 1 Diabetes
Miyazaki, B, Baldwin, J, Connard, J, Aceves, J, Alula, J, Berman, C, Ferris, J, Miller, D, Odugbesan, O, La Banca…
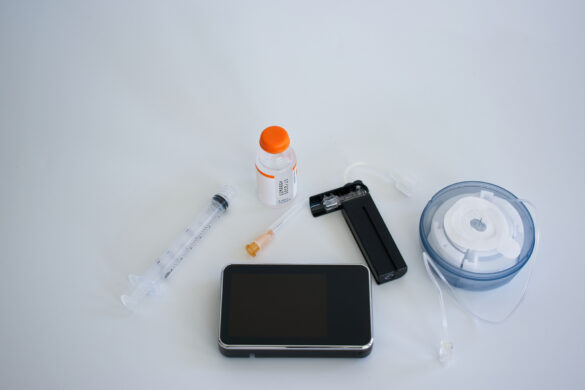
My Insulin Pump Malfunctioned — Now What?
Do you know what to do if your insulin pump malfunctions or fails? I can attest it’s a sobering moment…
How the iLet ACE Closed-Loop Insulin Pump is So User-Friendly
The iLet ACE insulin pump and iLet Dosing Decision Software from Beta Bionics were approved by the FDA on May…
Do You Currently Use a CGM?
Thank you for being a part of the T1D Exchange Online Community. We learn something new about life with type…
Life with the DIY Loop
I’ve lived with type 1 diabetes for the past 20 years, and in that time frame, managing my diabetes has…
Closed-Loop Study with Tandem “Artificial Pancreas” Showed Promising Results in Children
A 16-week, multi-center, randomized trial of over 100 children aged 6 to 13 at sites around the U.S. showed improved…
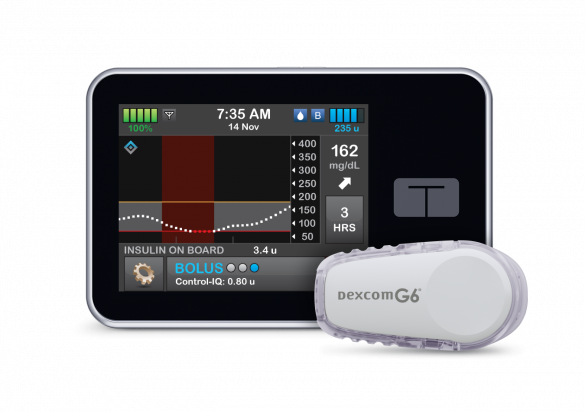
Tandem CCO Brian Hansen Discusses FDA Approval for Control IQ Algorithm with t:slim X2
Last month, the FDA approved the Tandem Diabetes Care update to its t:slim X2 insulin pump Control-IQ technology, clearing the way for the first automated and interoperable insulin dosing system on the U.S. market.