
Sign up for a new account.
And get access to
The latest T1D content
Research that matters
Our daily questions
Sign up by entering your info below.
Reset Your Password
Don't worry.
We will email you instructions to reset your
password.
We’re continuing to celebrate 6 years of the T1D Exchange Registry!
In honor of the anniversary of our online registry, we are showing some of the changes we’ve seen in type 1 diabetes (T1D) management and care since 2019.
Not already part of the T1D Exchange Registry? Join us!
In this article, we’re looking at changes in non-insulin medication usage and glucagon status among participants in the T1D Exchange Registry, comparing data from the registry’s early days (2019), “Then,” to the present (2025), “Now.”
We’ll look at the “Then” and “Now” for:
- Non-insulin medication usage rate
- GLP-1 usage rate
- Most commonly used GLP-1 medications
- Metformin usage rate
- SGLT-2 inhibitor usage rate
- Glucagon prescription status
- Most commonly prescribed glucagons
Want to see insights on insulin pump and CGM usage? Check out the first article in this series!
Here’s how various T1D medications used by our online registry participants have changed since it began in 2019.
Increasing Use of Non-Insulin Medications
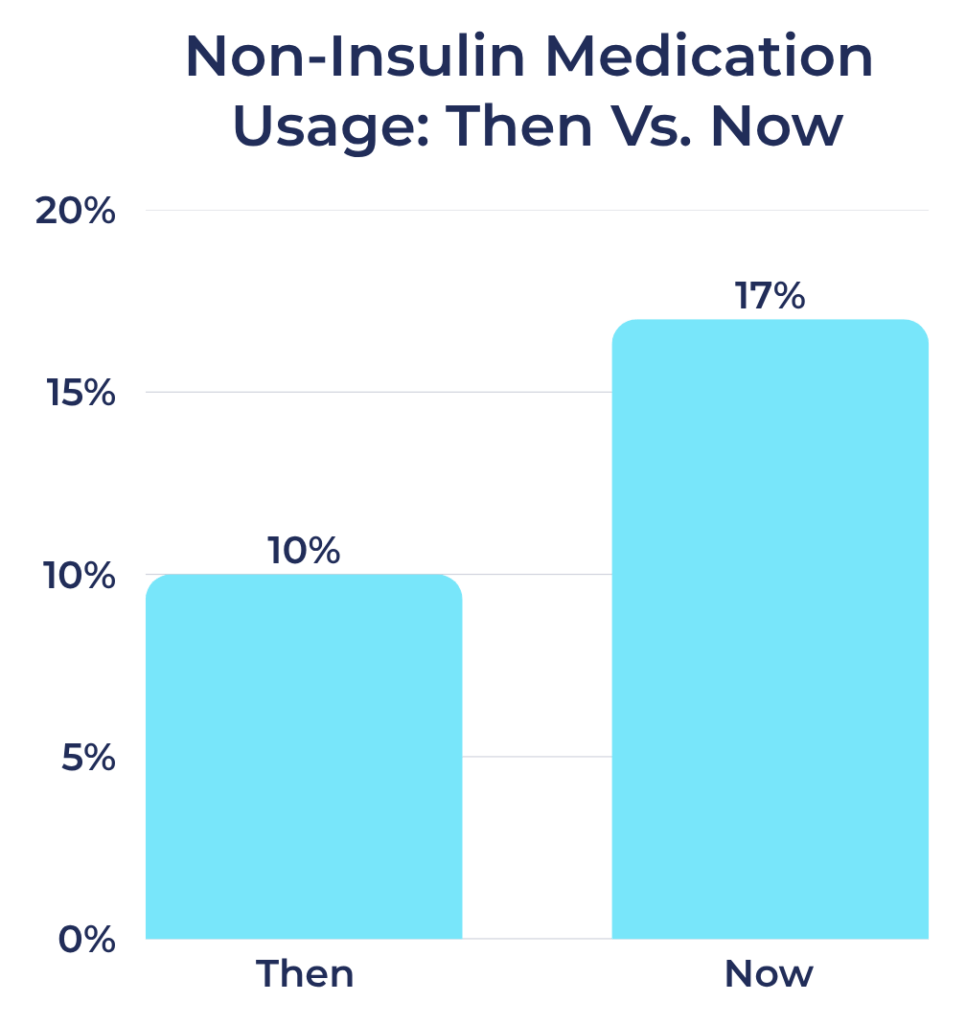
Non-Insulin Medication Use Among Registry Participants:
- Then: 10%
- Now: 17%
Many factors may have contributed to the rise in the use of type 2 diabetes medications among individuals with T1D over the past six years.
Increased awareness of these therapies and their benefits within the medical community, people living with diabetes, and on social media has likely facilitated the wider adoption of these treatments.
The Rise of GLP-1 Receptor Agonists
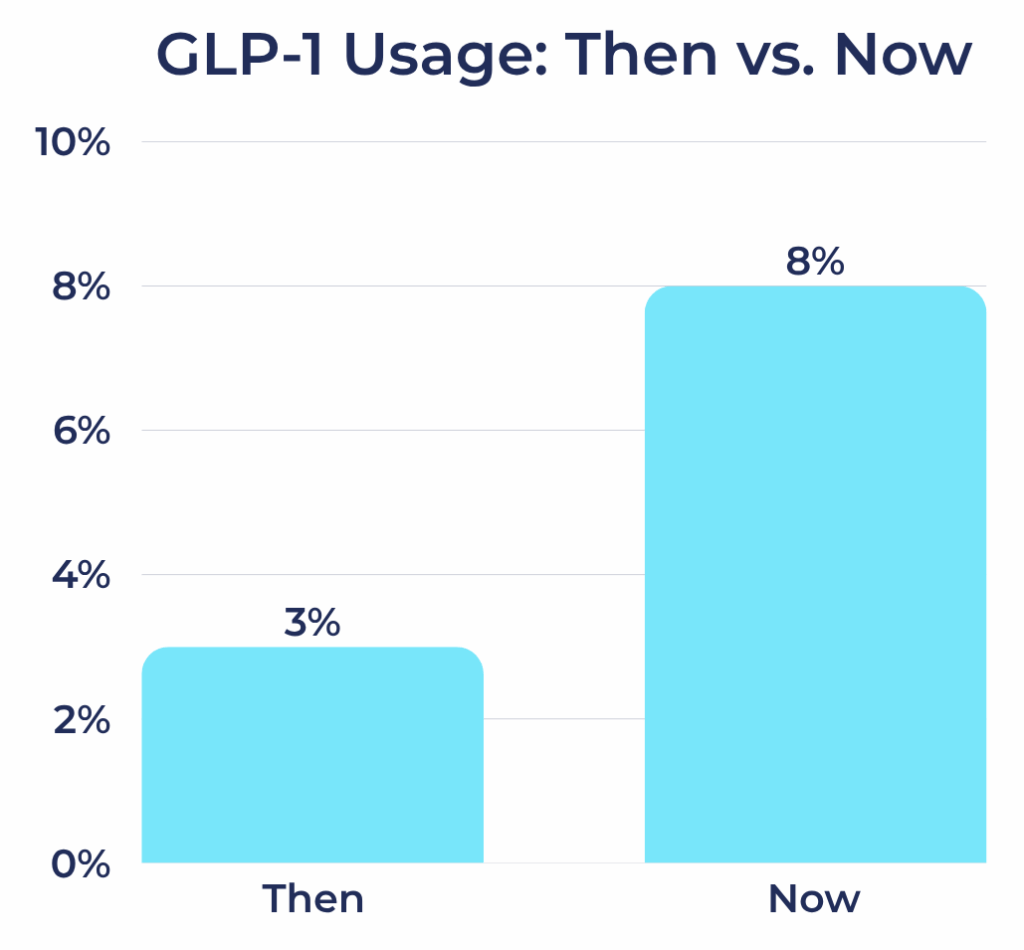
GLP-1 Receptor Agonist Usage Among Registry Participants
- Then: 3%
- Now: 8%
One significant change from 2019 to 2025 is the increase in GLP-1 receptor agonist usage among individuals with T1D in our registry.
Healthcare providers are increasingly willing to prescribe GLP-1 medications off-label for individuals with T1D, especially adults who also live with obesity or insulin resistance.

Top GLP-1 Medications Among Registry Participants in 2025
- Ozempic
- Mounjaro
- Wegovy
When we first launched our registry in 2019, GLP-1 use among people with T1D was rare, and we weren’t yet tracking which specific medications participants were using.
Fast forward to 2025, and that picture has changed. GLP-1s have become much more common in the T1D community. Ozempic tops the list among our registry participants, followed by Mounjaro and Wegovy.
Although these medications were initially developed to manage blood glucose, many people are drawn to their added benefits, especially appetite suppression and weight loss.
Metformin: A Steady Role
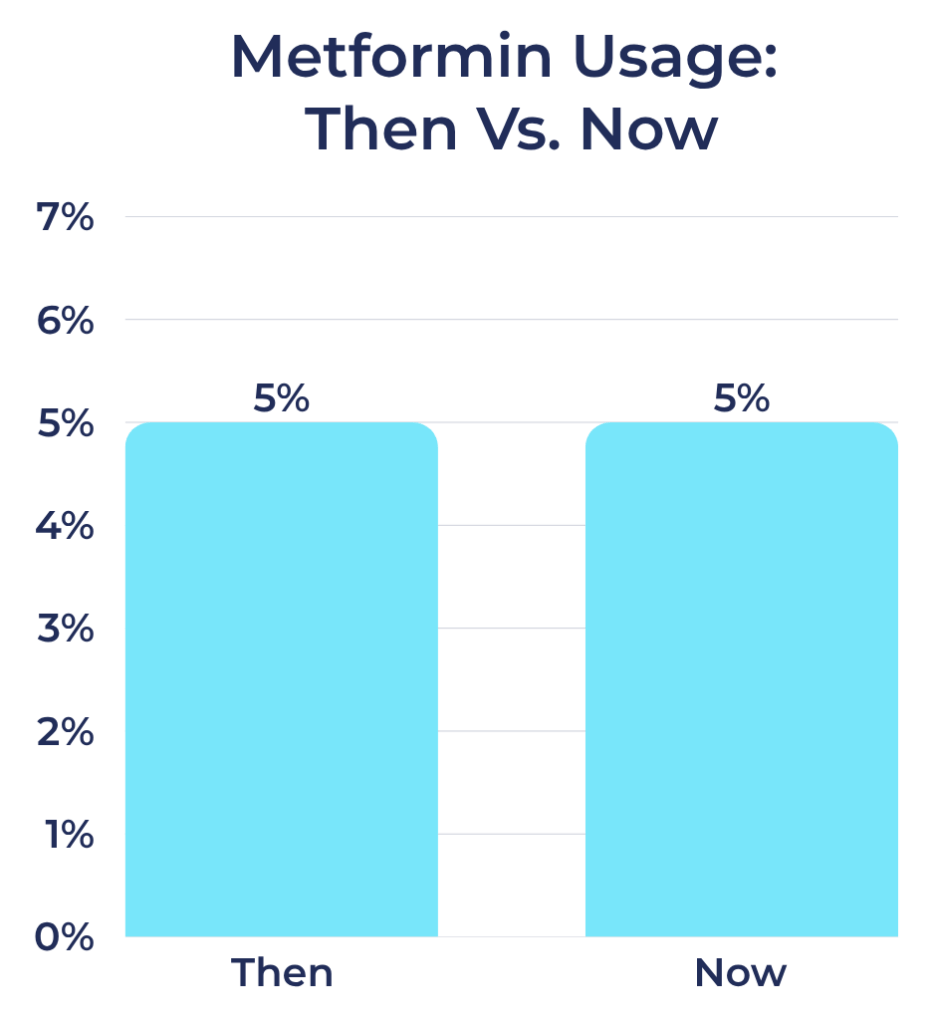
Metformin Usage Rates Among Registry Participants
- Then: 5%
- Now: 5%
Metformin use among participants in our registry hasn’t changed much in recent years. Though primarily used for type 2 diabetes, some clinicians prescribe it off-label for people with T1D who are dealing with insulin resistance or trying to lower their overall insulin needs.
While it can offer some modest benefits, its limited impact and lack of FDA approval for T1D may be part of the reason its use hasn’t really grown. While newer medications are making waves in the T1D space, metformin seems relatively stable.
SGLT-2 Inhibitors: Consistent Use and Continued Caution
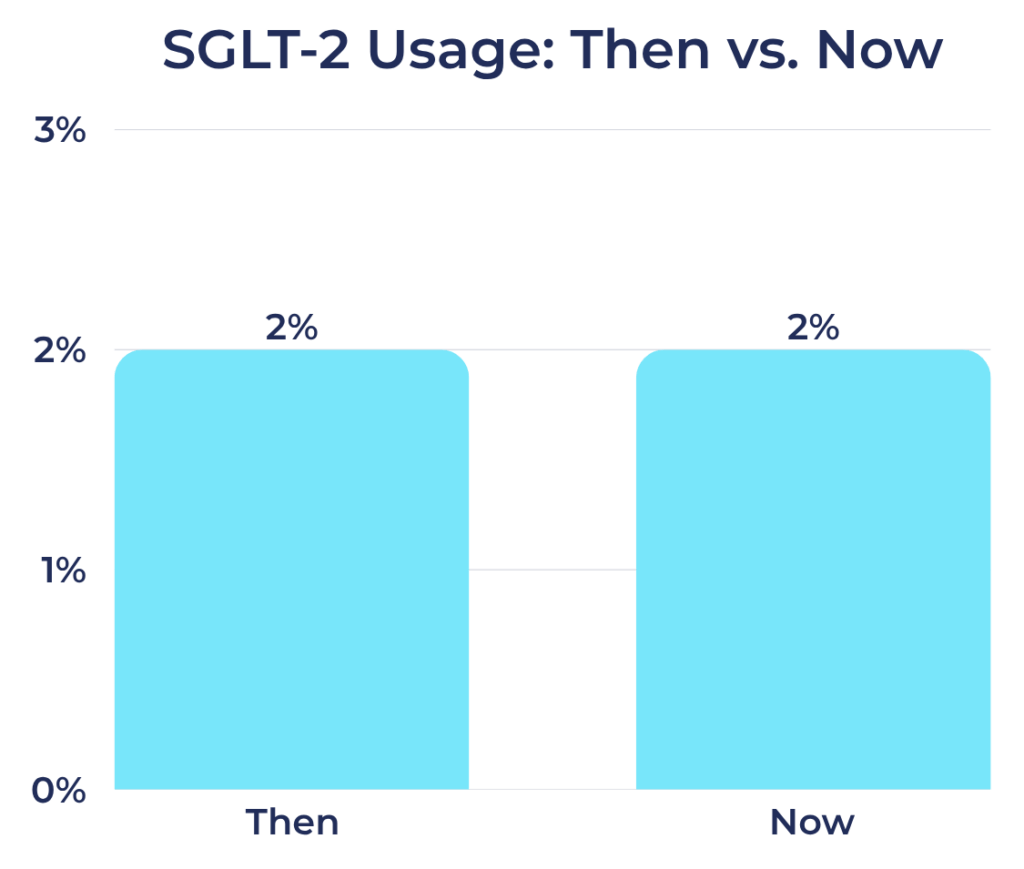
SGLT-2 Inhibitor Usage Among Registry Participants
- Then: 2%
- Now: 2%
SGLT-2 inhibitors reduce blood glucose by helping the kidneys to excrete excess glucose through urine. Their usage among individuals with T1D in our registry has remained unchanged from 2019 to 2025.
Although SGLT-2 inhibitors provide glucose-lowering and cardiovascular benefits in type 2 diabetes, they also pose a known risk of euglycemic DKA in individuals with T1D.
While some endocrinologists have cautiously prescribed these medications off-label, especially in people with obesity or cardiovascular risk, ongoing safety concerns have limited broader use.
Some studies and real-world evidence suggest SGLT-2 inhibitors may be helpful in highly selected individuals with T1D under close monitoring. However, the unchanged usage rate over six years may show that the diabetes community remains understandably cautious.
Changes in Glucagon Use and Preferences
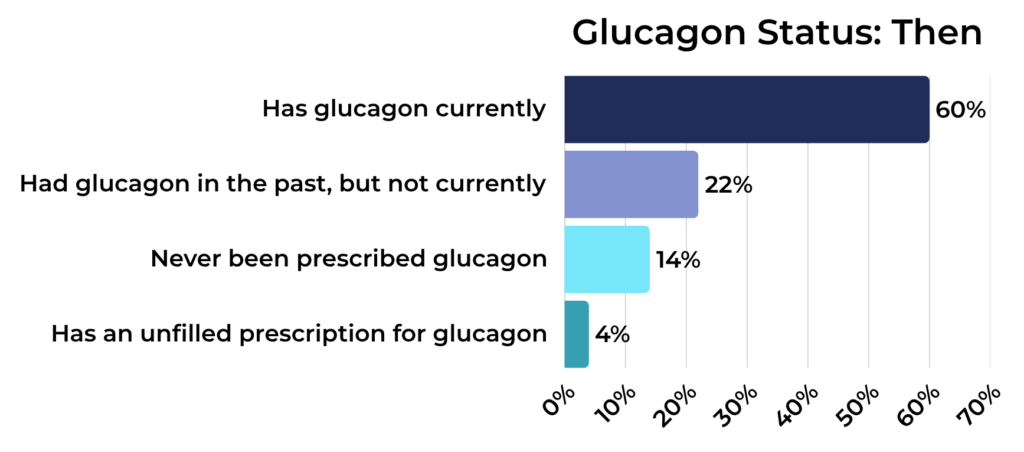
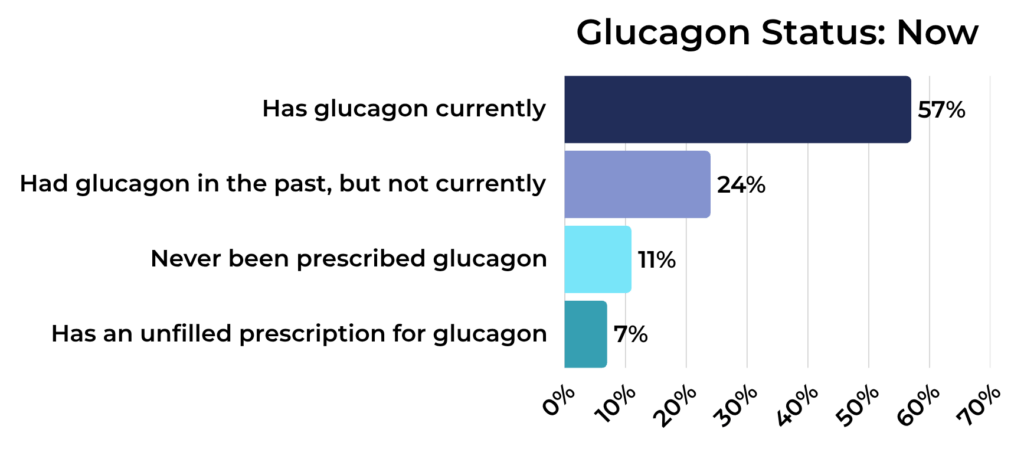
Glucagon Status Among Registry Participants:
Then:
- Has glucagon on hand: 60%
- Does not have glucagon on hand, but has been prescribed it in the past: 22%
- Has never been prescribed glucagon: 14%
- Has a current glucagon prescription, but has not filled it: 4%
Now:
- Has glucagon on hand: 57%
- Does not have glucagon on hand, but has been prescribed it in the past: 24%
- Has never been prescribed glucagon: 11%
- Has a current glucagon prescription, but has not filled it: 7%
Glucagon is a crucial emergency medication for severe hypoglycemia, and the data show that prescription rates have stayed relatively stable. In 2019 and 2025, most people with T1D in our registry had glucagon available.
While most either currently have glucagon or have had it prescribed in the past, a small but meaningful percentage report never having been prescribed it, and others report that they have a prescription they have a prescription for glucagon, but they haven’t filled it.
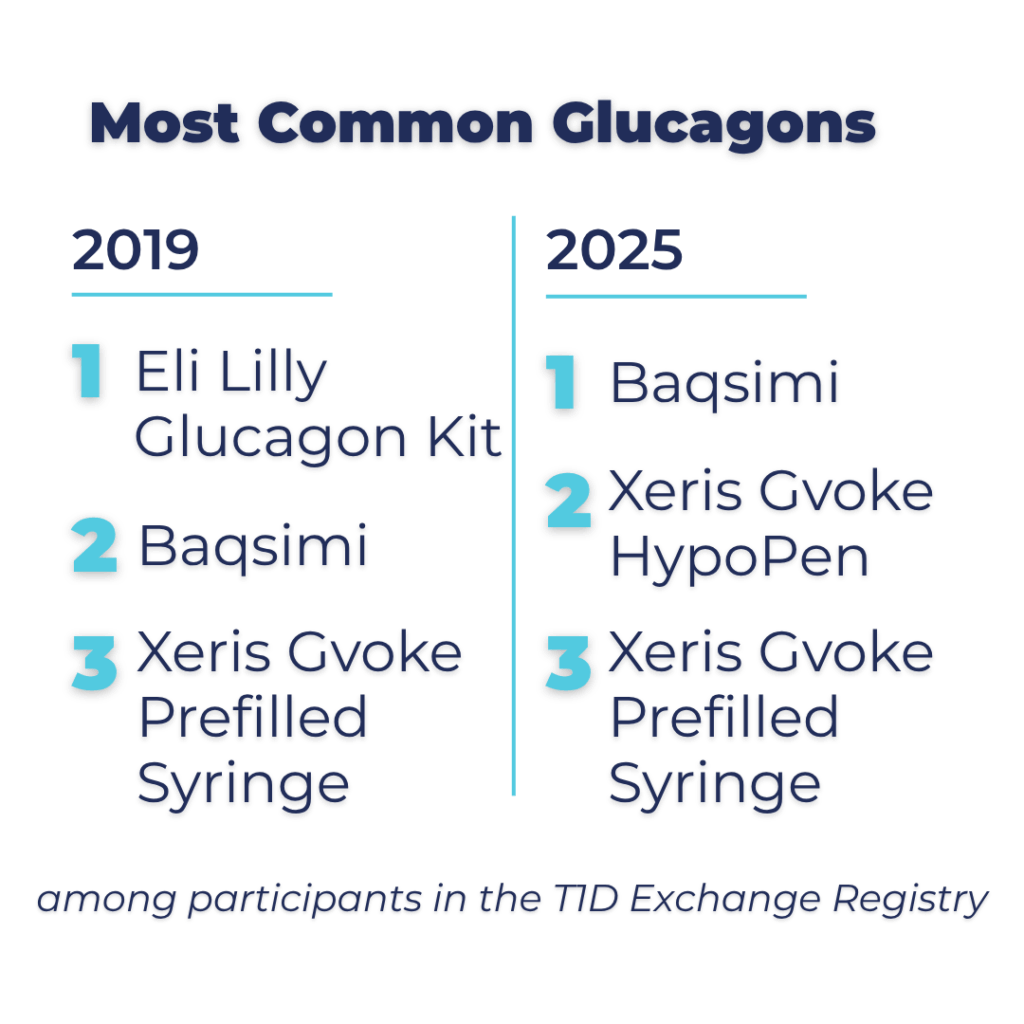
Top 3 Glucagons Among Registry Participants: Then Vs. Now
Then:
- Eli Lilly Glucagon Kit
- Baqsimi
- Xeris Gvoke Prefilled Syringe
Now:
- Baqsimi
- Xeris Gvoke HypoPen
- Xeris Gvoke Prefilled Syringe
The type of glucagon people use has changed significantly over the years. In 2019, the classic Eli Lilly emergency glucagon kit, which came in a red box and required an intramuscular injection, dominated the market.
Now, in 2025, newer glucagon options have become increasingly popular among our registry participants. Baqsimi, a needle-free nasal spray approved in 2019, has become more widely prescribed, thanks to its ease of use.
Other recent products have also gained ground, including the Xeris Gvoke HypoPen—an autoinjector that comes ready to use—and the Gvoke Prefilled Syringe, which eliminates the need for mixing.
This shift reflects a broader trend we’ve seen in previous T1D Exchange research: ease and simplicity matter when it comes to glucagon. The older intramuscular glucagon kits, which require mixing and large needles, are quickly being replaced by options that are faster and simpler to administer, and far less intimidating in an emergency.
Putting the Data into Context
Innovations in medication, easier-to-use delivery options, and evolving prescribing practices are all changing how people manage their T1D. On the other hand, safety concerns, regulatory limitations, and variable insurance coverage also influence which therapies gain traction in the community.
These insights come from real people living with T1D who are participating in research from the comfort of their homes through the T1D Exchange Registry.
If you are an adult with T1D or a caregiver of a child with T1D living in the U.S., join the T1D Exchange Registry to contribute to valuable insights like these!
Note: The data in this article does not imply cause and effect and shouldn’t be regarded as the final word on T1D. It represents only the individuals who have participated in the T1D Exchange Registry between 2019 and 2025, and it does not represent the entire T1D population.
While this data offers a limited, though valuable, perspective on the landscape of T1D, it should be interpreted cautiously.







